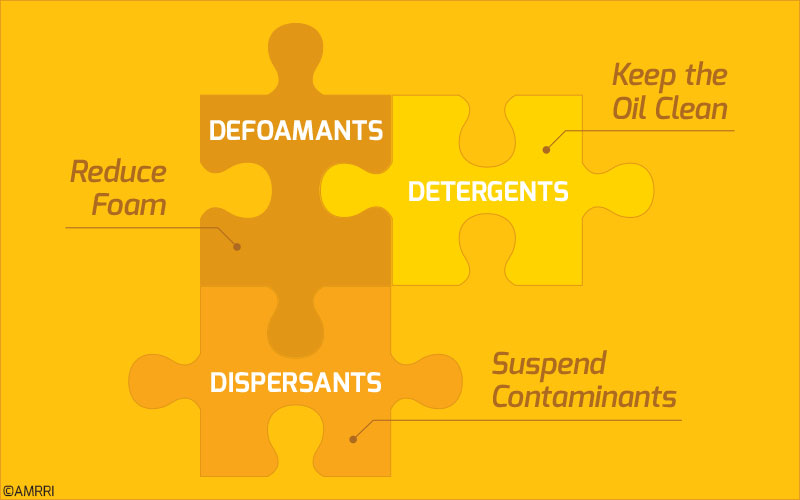
Traditionally, detergents were given their name as it was assumed that they provided cleaning properties to the oil, similar to laundry detergents. However, these metal-containing compounds also provide an alkaline reserve used to neutralize acidic combustion and oxidation by-products.
Due to their nature, these compounds disperse particulate matter, such as abrasive wear and soot particles, rather than removing them (in a cleaning action). There are four main types of detergents: phenates, salicylates, thiophosphate, and sulfonates4.
Calcium phenates are the most common type of phenate. They are formed by synthesizing alkylated phenols with elemental sulphur or sulphur chloride, followed by neutralization with metal oxides or hydroxides. These calcium phenates have good dispersant properties and possess a greater acid-neutralization potential.
Salicylates have additional antioxidant properties and a proven efficacy in diesel engine oil formulations. They are prepared through the carboxylation of alkylated phenols with subsequent metathesis into divalent metal salts. These products are then overbased with excess metal carbonate to form highly basic detergents.
Thiophosphonates are rarely used today as they are an overbased product.
Sulfonates generally have excellent anticorrosion properties. The neutral (or over-based) sulfonates have excellent detergent and neutralization potential. These neutral sulfonates are typically formed with colloidally dispersed metal oxides or hydroxides.
Calcium sulfonates are relatively cheap and have good performance. On the other hand, magnesium sulfonates exhibit excellent anticorrosion properties but can form hard ash deposits after thermal degradation, leading to bore polishing in engines. Barium sulfonates are not used due to their toxic properties.
Detergents in ATFs are used in concentrations of 0.1-1.0% for cleanliness, friction, corrosion inhibition, and reduction of wear3. However, these values are a bit higher in manual transmission fluids, at 0.0 – 3.0%. On the other hand, no detergents are required for axle lubricants!
Find out more in the full article, "Defoamants, Dispersants, and Detergents in Lubricants: A Complete Guide" featured in Precision Lubrication Magazine by Sanya Mathura, CEO & Founder of Strategic Reliability Solutions Ltd.