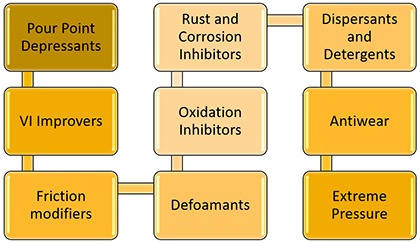
Each additive works differently to produce its function on the base oil and the overall finished lubricant. This section will explore how each of the lubricant additives works and some of the challenges they may experience.
Pour Point Depressants
As noted above, the pour point depressants help control the flow of the lubricant. This is achieved by modifying the wax crystals present in the lubricant’s base oil. At lower temperatures, the liquid usually has trouble being poured due to the presence of wax molecules in the base oil1.
There are two main types of pour point depressants, namely;
- Alkylaromatic polymers adsorb on the wax crystals as they form, thus preventing them from growing and adhering to each other. This effectively controls the crystallization process and ensures the lubricant can be poured.
- Polymethacrylates co-crystallize with wax to prevent crystal growth.
While these additives do not entirely prevent wax crystal growth, they lower the temperature at which these rigid structures are formed. These additives can achieve a pour point depression of up to 28°C (50°F); however, the common range is typically between 11-17°C (20-30°F).
Solubility thresholds may limit the use of this type of additive to achieve the desired effect on the base oil.
VI Improvers
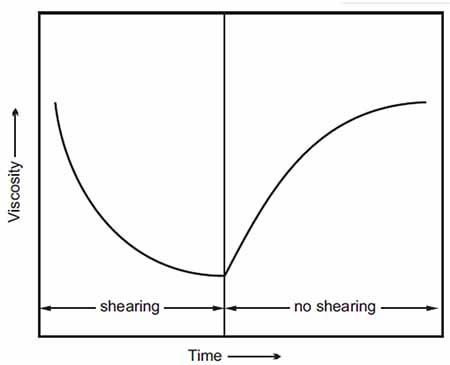
These additives are typically long-chain, high-molecular-weight polymers that change their configuration in the lubricant based on temperature4. When the lubricant is in a cold environment, these polymers adopt a coiled form to minimize the effect on viscosity. On the other hand, in a hot environment, they will straighten out, allowing the oil to produce a thickening effect.
While it is more desirable to use high molecular weight polymers (since they provide a better thickening effect), these long-chain molecules are also subject to degradation due to mechanical shearing. Therefore, a balance must be reached between the molecular weight and shear stable service condition.
Another challenge for formulators is to balance the polymer’s tendency to shear with the expected viscosity thickening due to oxidative processes and the viscosity thinning due to the dilution of fuel1.
Friction Modifiers
These usually compete with the antiwear and extreme pressure additives (and other polar compounds) for surface room. However, they become activated at temperatures when the AW and EP additives are not yet active. Thus, they form thin mono-molecular layers of physically adsorbed polar soluble products or tribochemical friction-reducing carbon layers, which exhibit a lower friction behavior than AW and EP additives2.
There are different groups of friction modifiers based on their function. Some are mechanically working FMs (solid lubricating compounds, e.g., Molybdenum disulfide, graphite, PTFE, etc.), adsorption layers forming FMs (e.g., fatty acid ester, etc.), tribochemical reaction layers forming FMs, friction polymer forming FMs and organometallic compounds.
Defoamants (Antifoam)
When foam forms in the lubricant, tiny air bubbles become trapped either at the surface or on the inside (called inner foam). Defoamants work by adsorbing on the foam bubble and affecting the bubble surface tension. This causes coalescence and breaks the bubble on the lubricant’s surface1.
For the foam that forms at the surface, called surface foam, defoamants with a lower surface tension are used. They are usually not soluble in base oil and must be finely dispersed to be sufficiently stable even after long-term storage or use.
On the other hand, inner foam, which is finely dispersed air bubbles in the lubricant, can form stable dispersions. Common defoamants are designed to control surface foam but stabilize inner foam2.
Oxidation Inhibitors
As noted above, antioxidants are usually deployed during the propagation phase to neutralize the scavenging radicals or decompose the hydroperoxides3. There are two main forms of antioxidants: primary and secondary antioxidants.
Primary antioxidants, also known as radical scavengers, remove radicals from oil. The most common types are amines and phenols.
Secondary antioxidants are designed to eliminate peroxides and form non-reactive products in the lubricant. Some examples include zinc dithiophosphate (ZDDP) and sulphurized phenols.
Mixed antioxidant systems also exist where two antioxidants have a synergistic relationship. One example is the relationship between phenols and amines, where phenols deplete early during oxidation while amines deplete later. Another example is using primary and secondary antioxidants to remove radicals and hydroperoxides.
Rust and Corrosion Inhibitors
Rust and Corrosion inhibitors are usually long alkyl chains and polar groups that can be adsorbed on the metal surface in a densely packed formation of hydrophobic layers.
However, this is a surface-active additive, and as such, it competes with other surface-active additives (such as antiwear or extreme pressure additives) for the metal surface. There are two main groups for corrosion additives: antirust additives (to protect ferrous metals) and metal passivators (for non-ferrous metals2).
Rus inhibitors have a high polar attraction to metal surfaces. They form a tenacious, continuous film that prevents water from reaching the metal surface. It must also be noted that contaminants can introduce corrosion into an oil, just as organic acids are produced.
Detergents and Dispersants
Detergents are polar molecules that remove substances from the metal surface, similar to a cleaning action. However, some detergents also provide antioxidant properties. The nature of a detergent is particularly important as metal-containing detergents produce ash (typically calcium, lithium, potassium, and sodium)1.
On the other hand, dispersants are also polar, and they keep contaminants and insoluble oil components suspended in the lubricant. They minimize particle agglomeration, which in turn maintains the oil’s viscosity (compared to particle coalescing, which leads to thickening). Unlike detergents, dispersants are considered ashless. They typically work at low operating temperatures.
Antiwear Additives
These are typically polar with long chain molecules that adsorb onto the metal surfaces to form a protective layer. This can reduce friction and wear under mild sliding conditions. Usually, these additives are formed from esters, fatty oils, or acids, which can only work at low or moderate levels of stress within the system.
The most common form of antiwear is ZDDP, which is used in engine or hydraulic oils. On the other hand, an ashless phosphorus type of antiwear also exists for systems that require that characteristic, and tricreysl phosphate is the usual choice.
Extreme Pressure Additives
Since extreme pressure additives only become active when higher temperatures or heavier loads are on a system, they have earned the name “Anti-scuffing additives.”
Unlike antiwear additives, extreme pressure additives react chemically with the sliding metal surfaces to form relatively insoluble surface films. This reaction only occurs at higher temperatures, sometimes between 180-1000°C, depending on the type of EP additive used1.
It must be noted that even with the presence of EP additives in a lubricant, there will still be some wear during the break-in period as the additives have yet to form their protective layers on the surfaces.
EP additives must also be designed for the system they protect as different metals have varying reactivity (EP additives designed for steel-on-steel systems may not be appropriate for bronze systems as they are not as reactive with bronze).
EP additives also contribute to polishing the sliding surfaces as they experience the most significant chemical reaction when the asperities are in contact and the localized temperatures are at their highest. They tend to be created from compounds containing sulphur, phosphorus, borate, chlorine, or other metals4.
Do Lubricant Additives Degrade Over Time?
As noted earlier, most additives can deplete over time as they get used up in their various functions. Antiwear and rust protection additives continuously coat the surfaces of the interfacing metals.
This can cause their initial concentrations to decrease over time until it reaches a point where the concentration of the additive is too low to offer any protection. In this case, it has not degraded but depleted.
In earlier years, there used to be prevalent issues with the separation of additives from the finished lubricant due to filtration. However, with the evolution of technology and better practices, this is no longer a common problem operators face.
In the past, operators would notice frequent clogging of their filters and subsequent reduction of additive concentrations, rendering the oil unprotected. It was common to notice additives settling to the bottom of a drum of oil after standing still for some time.
In essence, lubricant additives do not really degrade over time; rather, their concentrations get depleted, which assists in the lubricant degrading faster than a finished lubricant with higher additive concentrations.
Innovation and Future Trends for Additives
What does the future look like for additives within our industry? Will they go away completely?
From my estimations, we’re a long way from that happening. The lubricant industry has evolved over the years, with many advances from the chemical side, which has developed better-suited additives, and the OEM side, which has pushed the chemists to develop lubricant additives that can adapt to equipment changes.
OEMs are creating more components that can withstand higher temperatures, increased pressures, and more demanding environments. Lubricants must also be developed for this specific use, and additive technology will continue to evolve as these boundaries are pushed.
We are also being driven towards more environmentally friendly products, and additives are also on that list. Most of the metals used in the production of additives (such as EP or AW additives) are toxic to the environment, and alternatives are being discovered.
In the field of tribology, there has also been continued research into ways of reducing friction and wear. This is coupled with research into the interaction of varying surfaces and ways lubricants can effectively reduce the coefficient of friction, leading to increased energy efficiency and fuel efficiency in some cases.
Lubricant additives will be around for some time as everything that moves needs to be lubricated, and base oils do not have all the required properties to handle varying temperatures and other conditions that the machine encounters.
While their structure will change to adapt to provide a more environmentally friendly impact, their functions will also evolve based on their future requirements.
References
1 Bruce, R. W. (2012). Handbook of Lubrication and Tribology, Volume II Theory and Design, Second Edition. Boca Raton: CRC Press.
2 Mang, T., & Dresel, W. (2007). Lubricants and Lubrication – Second Completely Revised and Extended Edition. Weinheim: WILEY-VCH GmbH.
3 Livingstone, G., Wooton, D., & Ameye, J. (2015). Antioxidant Monitoring as Part of Lubricant Diagnostics – A Luxury or a Necessity?
4 Pirro, D. M., Webster, M., & Daschner, E. (2016). Lubrication Fundamentals – Third Edition Revised and Explained. Boca Raton: CRC Press.
Find out more in the full article, "Lubricant Additives: A Comprehensive Guide" featured in Precision Lubrication Magazine by Sanya Mathura, CEO & Founder of Strategic Reliability Solutions Ltd.