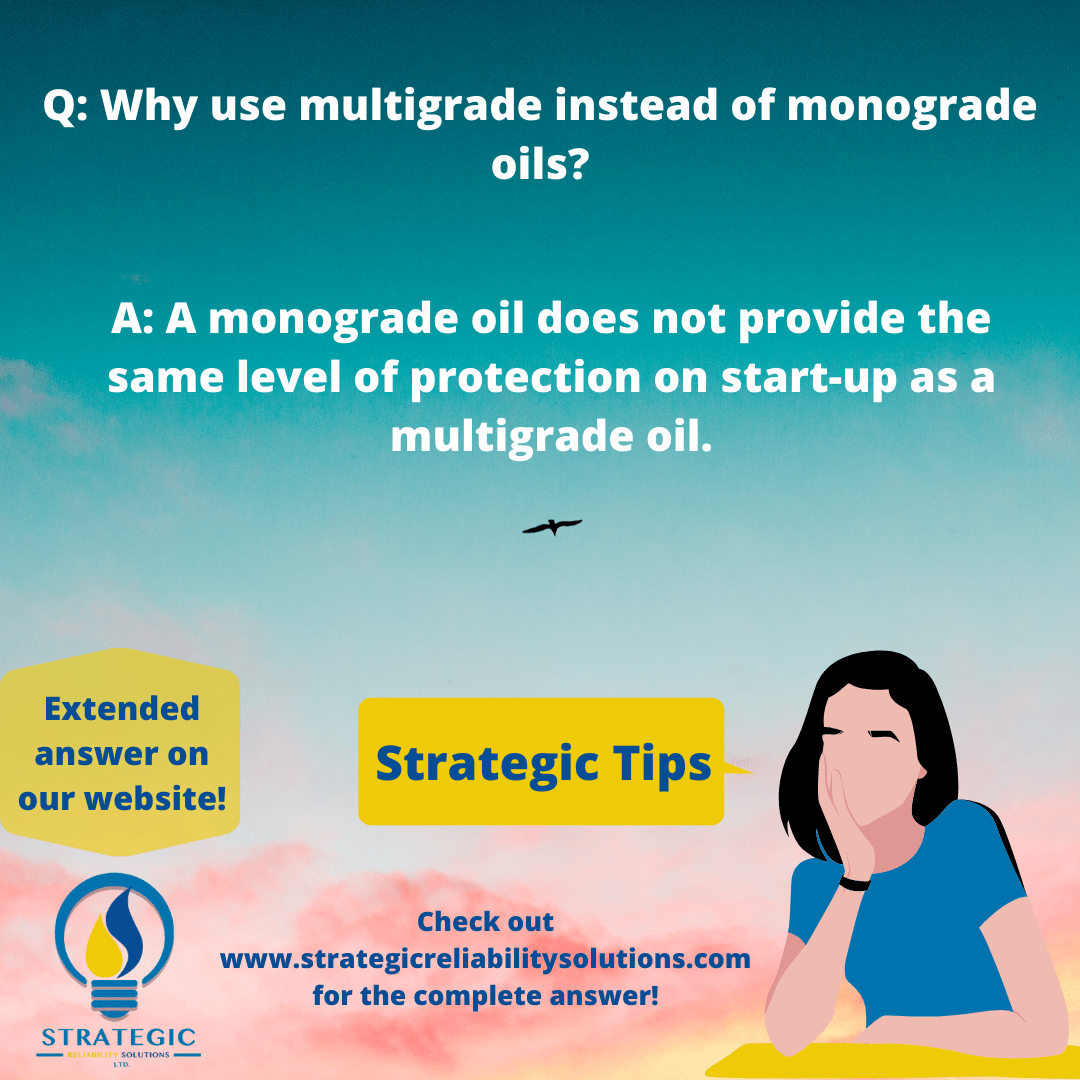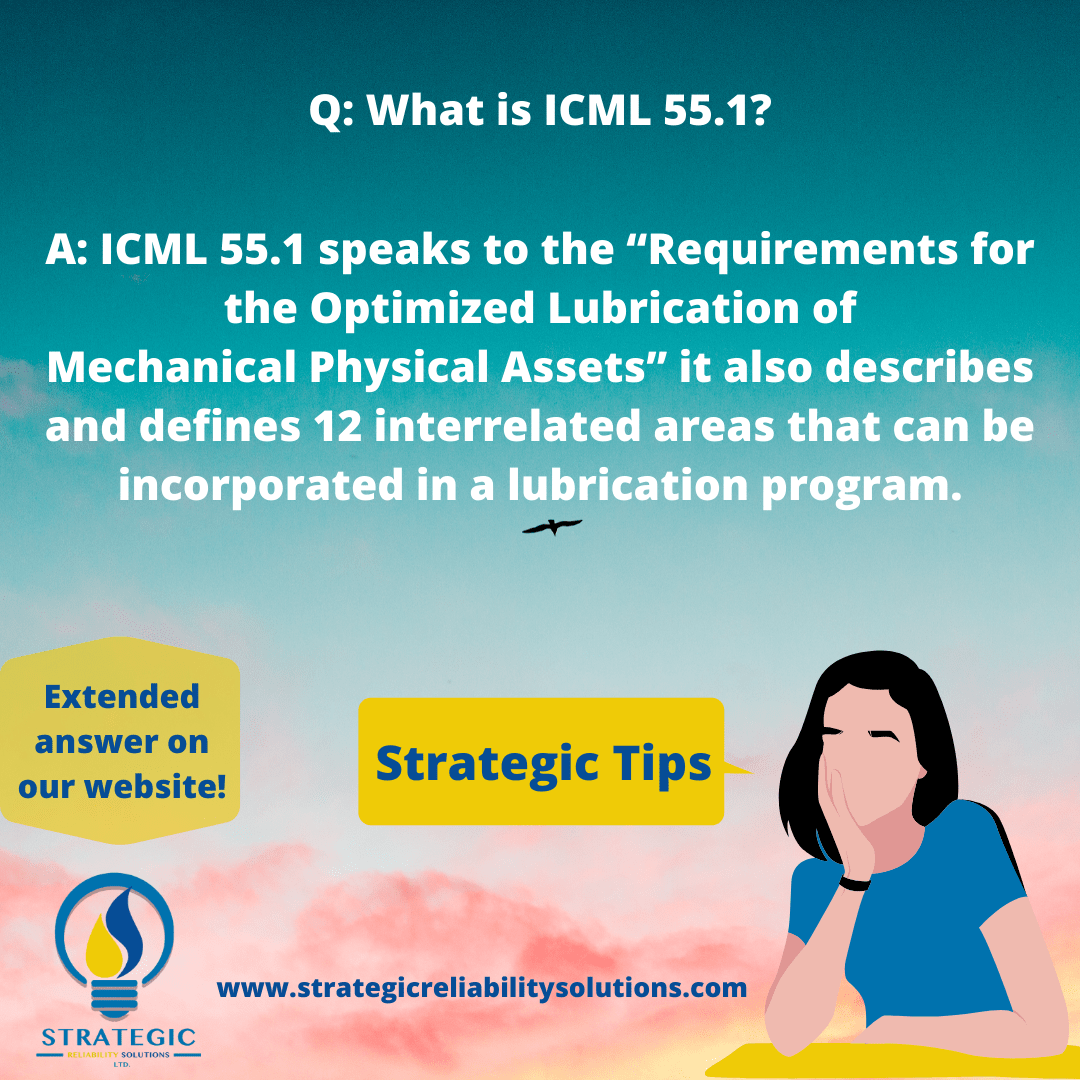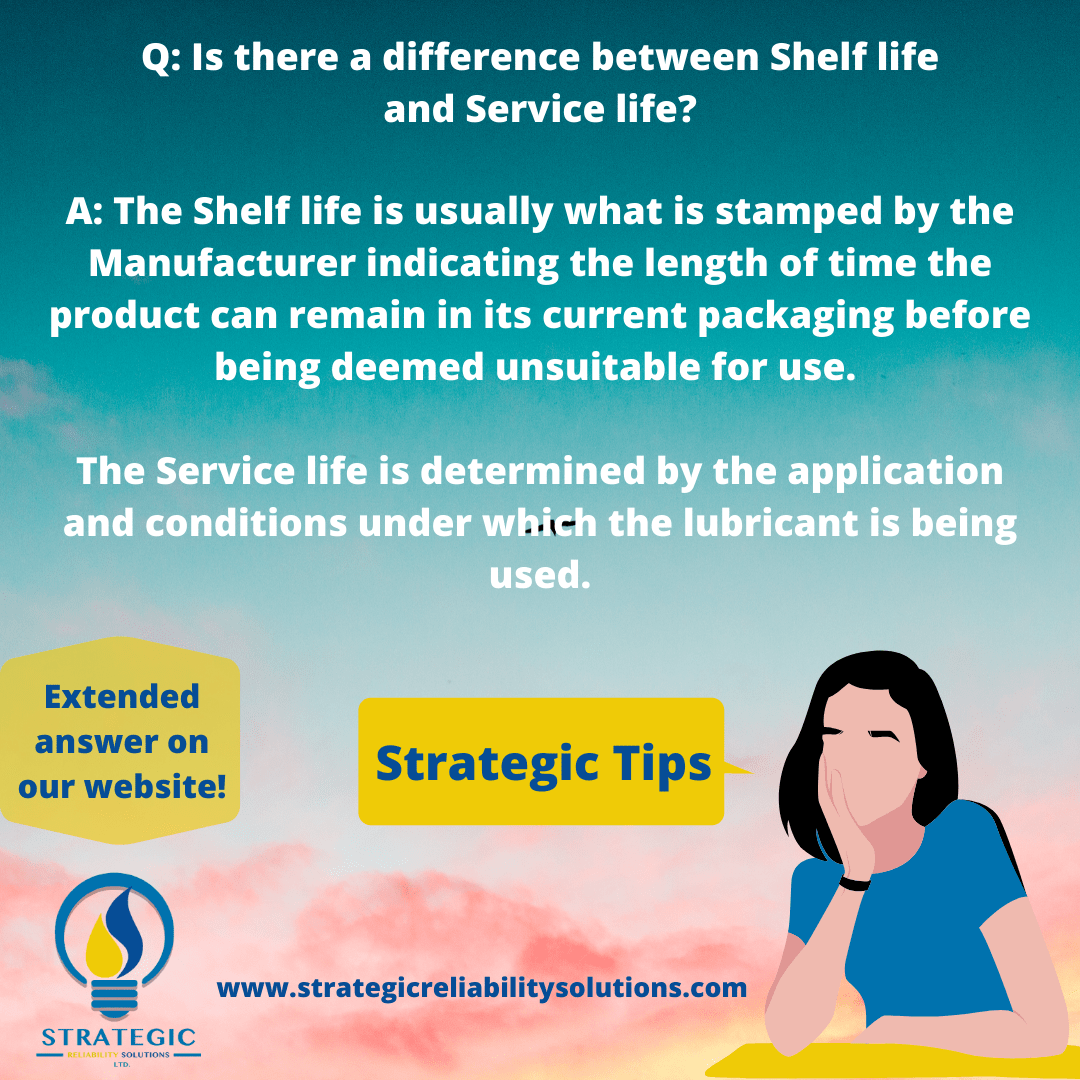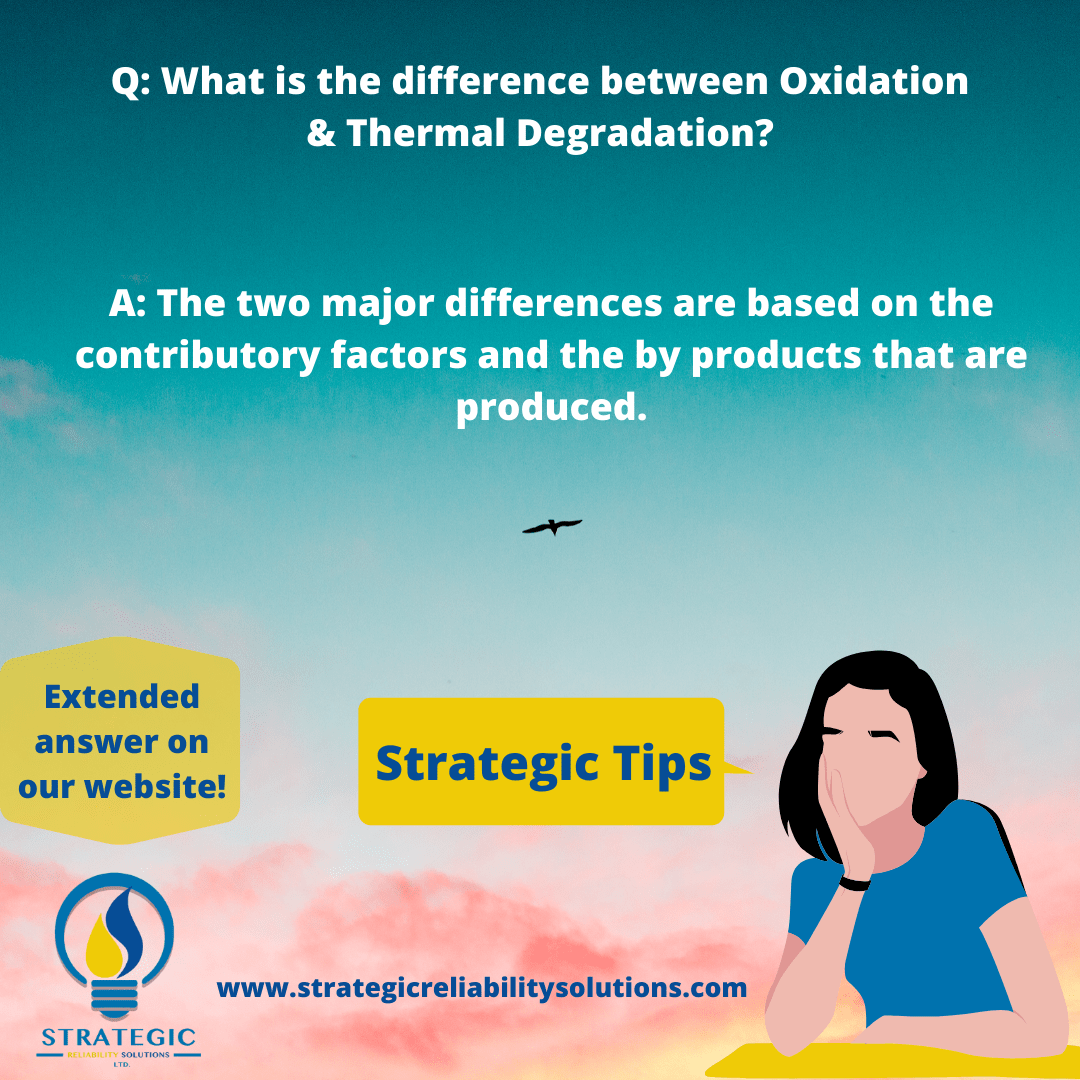
I can’t shut down the equipment but I know the oil has degraded significantly. What can I do?
Tough decisions!!!
There are times when production cannot be stopped such as when an order has to be fulfilled in a manufacturing facility. Before a decision is made, we need to understand the risks of not stopping production.
Can prolonged production cause a reduction in the overall quality of the final product or will it damage the equipment from working outside of its stipulated hours?
If we absolutely cannot shut down the equipment but the quality of oil has degraded, we need to firstly understand why the oil is degrading (especially if this is outside of its regular working hours).
Next, we need to identify which property of the oil has degraded, is it that the viscosity has increased / decreased, or the antioxidant levels have depleted significantly? By identifying the property that has degraded, we can choose the best way of replenishing this property.
Methods
There are a few methods that can be employed when trying to get the lubricant back to a healthy state however, as indicated above it is dependent on the property that has been degraded.
Cleanliness – if the ISO 4406 value has been increasing significantly this can hamper the performance of the lubricant. The clearances that the lubricant has to pass through can become blocked or the surfaces can experience an increased rate of wear.
One simple method of improving the cleanliness is through a kidney loop filtration system. This is an external system where the oil can be filtered through a filter cart and returned to the system.
Usually, this is a very effective method but one should investigate why the cleanliness values have become so high. Is it that the lubricant is being contaminated by the system, a process within the system or external factors?
Antioxidant levels – usually in turbines, this value decreases quickly especially if there is the presence of oxidation. Some users try to add antioxidants to their lubricant to increase the values. This is NOT recommended!!!
The composition of most turbine oils is 1% additive, 99% base oil. By adding any additive directly to the lubricant, we will be throwing the lubricant off balance and may induce other issues such as coagulation (clogged clearances) if the additive did not react well to the initial additives in the lubricant.
One of the easier ways of increasing the antioxidant levels without shutting down the machine is referred to as sweetening.
This process involves removing a percentage of the used oil (lubricant in the system) and then refilling the sump with new lubricant. The ratios can vary depending on the desired change in the antioxidant levels. It is important to note that the same lubricant should be used to ensure compatibility of the lubricants during the sweetening process.
Additionally, lab tests should be done frequently to monitor the changes in the antioxidant levels. The frequency of lab tests is highly dependent on the result turnaround time and budget available.
Written by Sanya Mathura, CEO & Founder of Strategic Reliability Solutions Ltd.